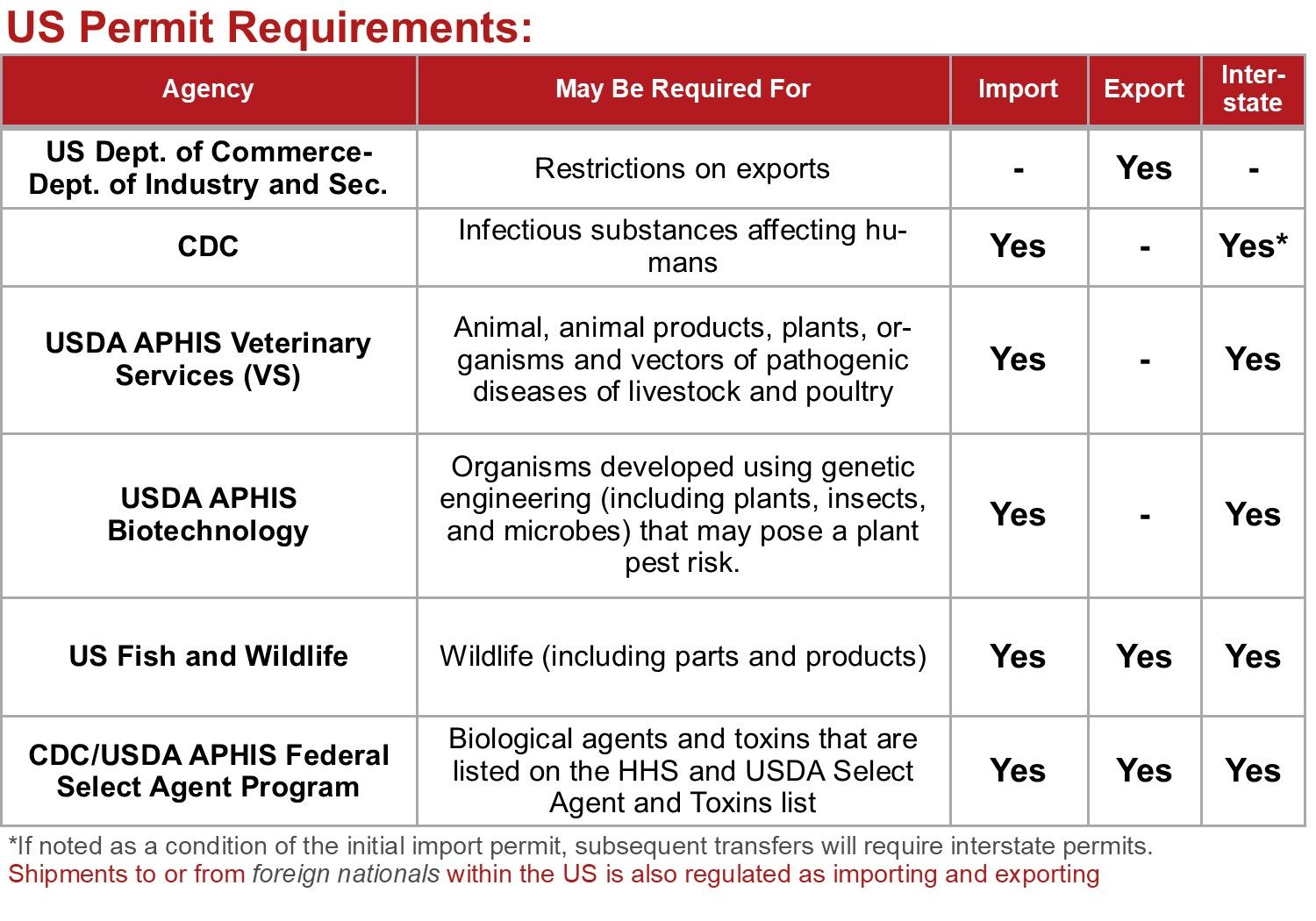
The import and export of chemical, biological and radioactive material may require additional permitting, to ensure that shipments clear customs in a proper and timely manner. Example U.S. regulatory agencies requiring permits may include:
- US Dept of Commerce - Bureau of Industry and Security (BIS)
- USDA Animal and Plant Health Inspection Service (APHIS) Organisms and Vectors Permits
- USDA Animal and Plant Health Inspection Service (APHIS) Biotechnology Permits
- US Fish and Wildlife Service Permits
- CDC Import Permit Program (IPP)
Tools are available on some regulatory websites to aid in deciding if a permit is required:
- CDC IPP Do I need a permit?
- CDC Import Permit Program IPP e-Tool
- CDC IPP Importer Certification Statement
- CDC eTool Decision Tree
- USDA/APHIS Organisms and Vectors Do I need a permit?
- See sections "What's New?", "Do I need an OV permit?", and "VS-regulated livestock and poultry pathogens", "Guidelines for No Import Permit Required", and "Guideline 1125: Guideline for No Interstate Transport Permit Required".
- Veterinary Services Permitting Assistant
Some materials require additional approval before they can be transferred by the original permit holder to an additional recipient. For example, the CDC requires additional approval for the following materials suspected or know to contain the following infectious biological agents:
- Mycobacterium tuberculosis
- Coronaviruses (SARS-CoV-2, MERS-CoV)
- Influenza viruses (H2N2, H6N1, low pathogenic avian H7N9)
- Viral hemorrhagic fevers (e.g., Tick-borne encephalitis viruses – Central European subtypes, Old World hantaviruses that cause hemorrhagic fever with renal syndrome (HFRS))
- Mpox (clade II) (formerly known as: Monkeypox – West African clade)
- Poliovirus (serotypes 1, 2, 3)
The permit requirements and process can be confusing but EHS Biosafety can help. Contact EHS for additional assistance.

